Key Market Insights
- More than 100 industry and non-industry players are currently evaluating the potential of over 190 TCR-based immunotherapies for the treatment of various oncological and non-oncological disorders
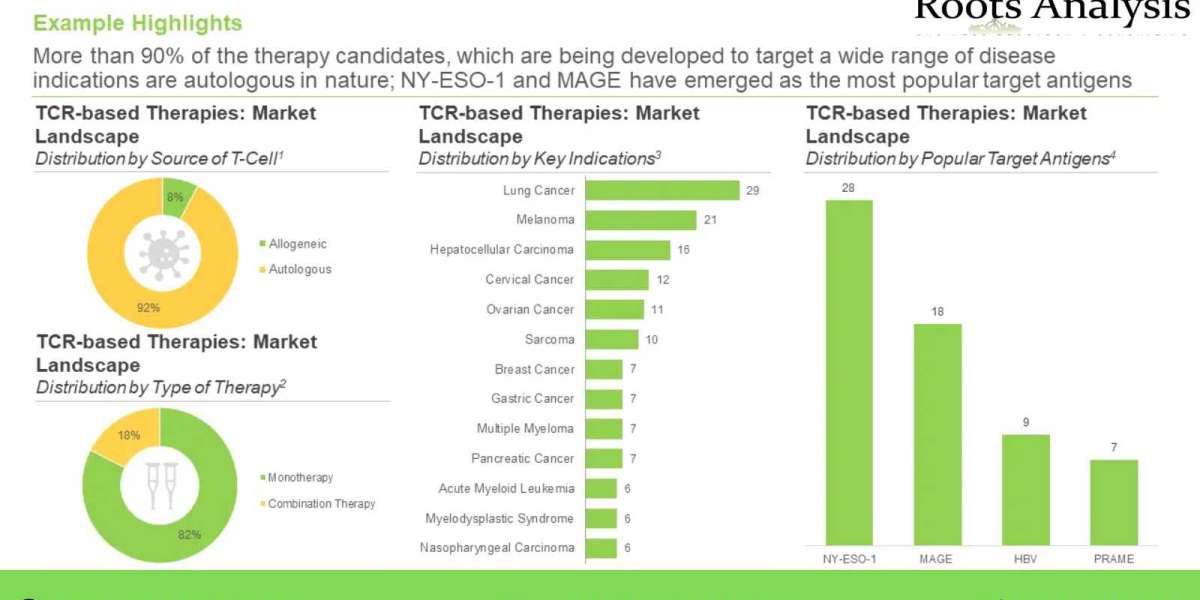
- More than 90% of the therapy candidates, which are being developed to target a wide range of disease indications are autologous in nature; NY-ESO-1 and MAGE have emerged as the most popular target antigens
- In the last 10 years, close to 110 clinical trials have been registered across different geographies for the evaluation of TCR-based therapies; extensive efforts are underway to improve the successive generations of such therapies
- Close to 60 scientists from renowned universities are presently involved in the clinical development of TCR therapies; majority of these KOLs are primarily based in the US and China
- Close to 200 players claim to have the required capabilities to manufacture different types of cell therapies; such firms also offer a wide range of services across different stages of product development
- A growing interest in this field is reflected from the increase in the partnership activity, involving both international and indigenous stakeholders; majority of such deals were signed between players based in North America
- Several investors, having realized the opportunity within this upcoming segment of T-cell immunotherapy, have invested USD 11 billion, across 140 instances, since 2007
- More than 75 patents have been filed / granted by various stakeholders in order to protect the intellectual property generated within this field
- With a growing focus on the development pipeline and encouraging clinical results, the TCR Therapy market is anticipated to witness an annualized growth rate of 51%, in the next decade
Table of Contents
- PREFACE
1.1. Introduction
1.2. Key Market Insights
1.3. Scope of the Report
1.4. Research Methodology
1.5. Key Questions Answered
1.6. Chapter Outlines
- EXECUTIVE SUMMARY
- INTRODUCTION
3.1. Chapter Overview
3.2. Pillars of Cancer Therapy
3.3. Overview of Immunotherapies
3.4. Fundamentals of Cancer Immunotherapy
3.5. Classification of Cancer Immunotherapies
3.6. T-Cell Immunotherapies
3.7. T-Cell Receptor (TCR)-based Cell Therapy
3.8. Concluding Remarks
- TCR-BASED THERAPIES: MARKET LANDSCAPE
4.1. Chapter Overview
4.2. TCR-based Therapies: Overall Market Landscape
4.3. TCR-based Therapies: Overall Developer Landscape
- POPULAR TARGET ANTIGEN ANALYSIS
5.1. Chapter Overview
5.2. Competitive Analysis: Popular Target Antigens of TCR-based Therapies
- CLINICAL TRIAL ANALYSIS
6.1. Chapter Overview
6.2. Scope and Methodology
6.3. TCR-based Therapies: Clinical Trial Analysis
- KEY OPINION LEADERS
7.1. Chapter Overview
7.2. Assumptions and Key Parameters
7.3. Methodology
7.4. TCR-based Therapies: Key Opinion Leaders
- TCR-BASED THERAPY PROFILES
8.1. Chapter Overview
8.2. Kimmtrak® / IMCgp100 / Tebentafusp (Immunocore)
8.3. GSK3377794 / NY-ESO-1C259 T-cells / Letetresgene Autoleucel (GlaxoSmithKline)
8.4. ADP-A2M4 / Afamitresgene Autoleucel / Afami-cel (Adaptimmune Therapeutics)
8.5. JTCR016 (Juno Therapeutics)
8.6. TBI-1301 (Takara Bio)
8.7. MDG1011 (Medigene)
- PARTNERSHIPS AND COLLABORATIONS
9.1. Chapter Overview
9.2. Partnership Models
9.3. TCR-based Therapies: Partnerships and Collaborations
- FUNDING AND INVESTMENT ANALYSIS
10.1. Chapter Overview
10.2. Types of Funding
10.3. TCR-based Therapies: Funding and Investment Analysis
- PATENT ANALYSIS
11.1. Chapter Overview
11.2. Scope and Methodology
11.3. TCR-based Therapies: Patent Analysis
- CASE STUDY: CELL THERAPY MANUFACTURING
12.1. Chapter Overview
12.2. Overview of Cell Therapy Manufacturing
12.3. Cell Therapy Manufacturing Models
12.4. Scalability of Cell Therapy Manufacturing Processes
12.5. Types of Cell Therapy Manufacturers
12.6. Key Challenges Related to Manufacturing of Cell Therapies
12.7. Important Factors for Cell Therapy Manufacturing
12.8. Automation of Cell Therapy Manufacturing Processes
12.9. Cell Therapy Manufacturing Supply Chain
12.10. Comparison of Player Having In-House Capabilities and Contract Manufacturers
12.11. Regulatory Landscape
12.12. Future Perspectives
- COST PRICE ANALYSIS
13.1. Chapter Overview
13.2. Factors Contributing to the High Price of Cell / Gene Therapies
13.3. Pricing Models for T-Cell Immunotherapies
13.4. Reimbursement related Considerations for T-cell Immunotherapies
- MARKET FORECAST AND OPPORTUNITY ANALYSIS
14.1. Chapter Overview
14.2. Scope and Limitations
14.3. Key Assumptions and Forecast Methodology
14.4. Global TCR-based Therapies Market, 2022-2035
- PROMOTIONAL ANALYSIS
15.1. Chapter Overview
15.2. Channels Used for Promotional Campaigns
15.3. Kimmtrak: Promotional Analysis
- COMPANY PROFILES
16.1. Chapter Overview
16.2. Adaptimmune Therapeutics
16.3. Alaunos Therapeutics
16.4. Company Profiles
16.5. Bristol Myers Squibb
16.6. Cellular Biomedicine Group
16.7. Gilead Sciences
16.8. Cellular Biomedicine Group
16.9. GlaxoSmithKline
16.10. Immatics
16.11. Immunocore
16.12. Lion TCR
16.13. Takara Bio
16.14. Zelluna immunotherapy
- CONCLUDING REMARKS
- EXECUTIVE INSIGHTS
18.1. Chapter Overview
18.2. Celyad
18.3. Kite Pharma
18.4. Lion TCR
18.5. TxCell
- APPENDIX 1: TABULATED DATA
- APPENDIX 2: LIST OF COMPANIES AND ORGANIZATIONS
To view more details on this report, click on the link:
https://www.rootsanalysis.com/reports/tcr-based-therapies-market.html
Learn from experts: do you know about these emerging industry trends?
Investment Opportunities in Women’s Digital Health
Genotoxicity Testing: Unlocking the Future Safety Assessment Opportunities
About Roots Analysis
Roots Analysis is a global leader in the pharma / biotech market research. Having worked with over 750 clients worldwide, including Fortune 500 companies, start-ups, academia, venture capitalists and strategic investors for more than a decade, we offer a highly analytical / data-driven perspective to a network of over 450,000 senior industry stakeholders looking for credible market insights.
Learn more about Roots Analysis consulting services:
Roots Analysis Consulting - the preferred research partner for global firms
Contact:
Ben Johnson
+1 (415) 800 3415