Roots Analysis has announced the addition of “ADC Contract Manufacturing Market (5th Edition), 2022-2035” report to its list of offerings.
The report features report features an extensive study of the current market landscape and the likely future potential associated with the ADC contract manufacturing market, over the next decade. The study includes an in-depth analysis, highlighting the capabilities of contract services providers engaged in this domain. Amongst other elements, the report features:
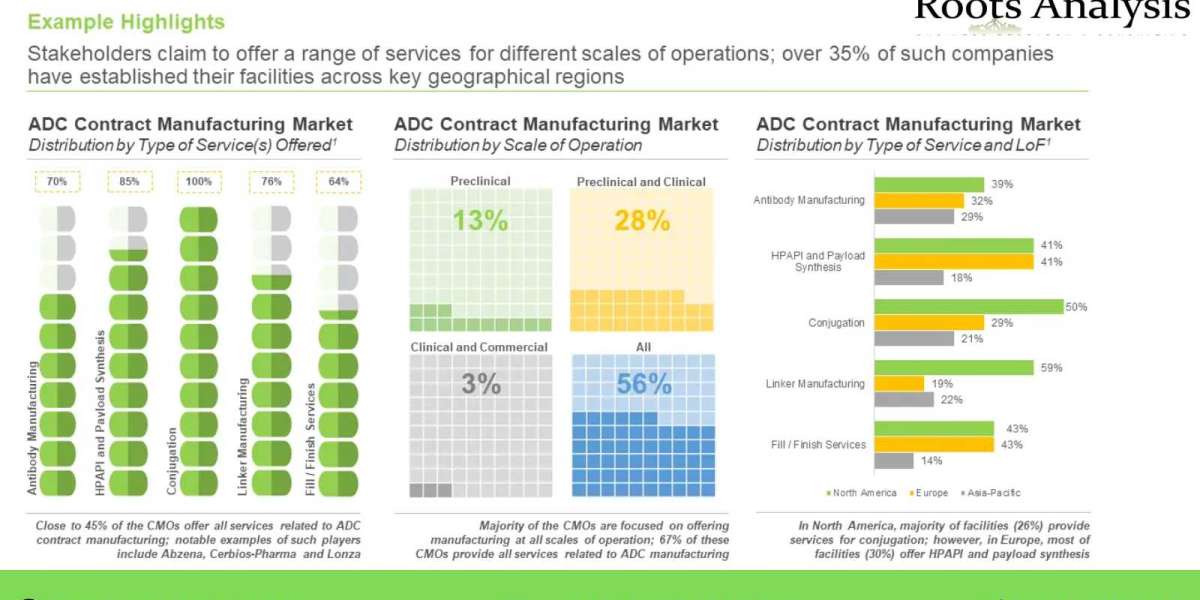
- A detailed overview of the overall market landscape of players engaged in the contract manufacturing of ADCs, based on several relevant parameters, such as company size, year of establishment, location of headquarters, type of service(s) offered (antibody manufacturing, HPAPI and payload synthesis, linker manufacturing, conjugation and fill-finish), other ADC service(s) offered (proof-of-concept studies / process development and scale-up / analytical development), scale of operation (preclinical, clinical and commercial) and location of manufacturing facilities. In addition, it provides details on the antibody contract manufacturers, HPAPI / cytotoxic payload contract manufacturers and biologics fill / finish service providers engaged in this domain.
- A detailed competitiveness analysis of ADC manufacturers, based on manufacturing strength (on the basis of scale of operation and number of ADC manufacturing facilities), service strength (based on the number of ADC services offered, number of additional services offered and location of ADC manufacturing facilities), supplier strength (in terms of employee count and years of experience in this field).
- Elaborate profiles of ADC contract manufacturers (shortlisted on the basis of competitiveness analysis). Each profile provides a brief overview of the company, its financial information, along with details on its ADC manufacturing capabilities, location of facilities, recent developments, and a comprehensive future outlook.
- A detailed analysis of the various expansion initiatives undertaken by service providers engaged in this domain, during the period 2012-2022, along with information on several relevant parameters, such as year of expansion, type of expansion (capacity expansion and new facility), type of service(s) offered (manufacturing services, analytical / development services and fill / finish services), location of expanded facility, scale of operation (preclinical, clinical and commercial) and most active players (in terms of number of instances).
- An analysis of the recent partnerships inked between various players engaged in this domain. Additionally, it includes a brief description of the various types of partnership models (manufacturing agreements, product development agreements, research agreements, service alliance, acquisitions, product development and manufacturing agreements, licensing agreements, and others) that have been adopted by stakeholders engaged in this domain.
- A qualitative analysis highlighting the various factors that need to be taken into consideration by ADC developers, while deciding whether to manufacture their respective products in-house or outsource the manufacturing to a contract service provider.
- A detailed discussion on various steps (antibody manufacturing, payload manufacturing, linker manufacturing, conjugation and fill / finish) involved in the manufacturing of ADCs, along with information on the cost requirements across each stages.
- An estimate of the overall ADC manufacturing / bioconjugation capacity (in kilograms) of contract manufacturers of contract manufacturers based on information provided by various stakeholders in the public domain. The analysis highlights the distribution of global capacity by company size (small, mid-sized and large), key geographical regions (North America, Europe and Asia-Pacific) and key players (in terms of highest bioconjugation capacity).
- A detailed overview of the ADCs that are either approved or under development (clinical and preclinical), along with information on their current phase of development (marketed, clinical and preclinical / discovery stage), target indication(s), target antigen, antibody origin, antibody isotype, type of payload and type of linker.
- A review of the evolution of ADC conjugation technologies, highlighting the various types of approaches that have been adopted in the past, and the different generations of linkers. It also highlights the competition between contemporary technology platforms.
- An analysis of completed, ongoing and planned clinical studies, based on several relevant parameters, such as number of trials registered, trial phase, trial status, target indication, type of sponsor / collaborator and number of patients enrolled.
- An informed estimate of the annual demand for ADC products (in kilograms), taking into account commercial, as well as clinical scale requirements, based on relevant parameters, such as target patient population, dosing frequency and dose strength of approved products and clinical stage candidates.
- An in-depth analysis of over 80 ADC based therapy developers that are likely to partner with contract service providers engaged in this domain, based on several relevant parameters, such as developer strength (on the basis of company size and its experience), pipeline strength and maturity (on the basis of number of drugs in pipeline, their stage of development and type of target indication) and manufacturing capabilities.
- A detailed regional capability assessment framework, which compares the key geographies, based on a number of parameters, such as the number of ADC contract manufacturers, number of ADC manufacturing facilities, number of facility expansions, installed ADC capacity, number of registered clinical trials and demand for ADCs in that particular geographical region.
- A proprietary 2×2 representation, highlighting the current market scenario (in terms of existing competition and growth opportunities) across emerging and established market segments.
- A discussion on affiliated trends, key drivers and challenges, under a SWOT framework, featuring a Harvey ball analysis, highlighting the relative impact of each SWOT parameter on the overall ADC contract manufacturing market.
- A detailed market forecast, featuring analysis of the current and projected future opportunity across key market segments (listed below)
- Scale of Operation
- Commercial
- Clinical
- Phase of Development
- Phase III
- Phase II
- Phase I
- Type of Component Manufacturing
- Antibody manufacturing
- HPAPI / Cytotoxic Payload and Linker Manufacturing
- Conjugation
- Fill / Finish
- Target Indication
- Solid Tumors
- Hematological Malignancies
- Others
- Type of Payload Used
- Maytansinoid
- Auristatin
- Camptothecin
- PBD
- Others
- Type of Linker Used
- SMCC
- VC
- Malemide
- Peptide Linker
- Others
- Type of Antibody Origin
- Humanized
- Chimeric
- Murine
- Human
- Others
- Type of Antibody Isotype
- IgG1
- Others
- Key Geographical Regions
- North America
- Europe
- Asia-Pacific
- Rest of the World
The opinions and insights presented in the report were also influenced by discussions held with key stakeholders in the industry. The report features detailed transcripts of interviews held with the following industry stakeholders:
- Aldo Braca (Chief Executive Officer, BSP Pharmaceuticals) and Giorgio Salciarini (Technical Business Development Manager, BSP Pharmaceuticals)
- Christian Rohlff (Chief Executive Officer Founder, Oxford BioTherapeutics)
- John Burt (ex-Chief Executive Officer, Abzena)
- Sasha Koniev (Chief Executive Officer Co-Founder, Syndivia)
- Denis Angioletti (Chief Commercial Officer, Cerbios-Pharma)
- Wouter Verhoeven (Chief Business Officer, NBE-Therapeutics)
- Takashi Owa (Chief Innovation Officer, Eisai) and Toshimitsu Uenaka (Executive Director, Eisai)
- Anthony DeBoer (Director, Business Development, Synaffix)
- Christian Bailly (ex-Director of CDMO, Pierre Fabre)
- Jennifer L. Mitcham (Director, Business Development, Catalent Pharma Solutions) and Stacy McDonald (ex-Group Product Manager, Catalent Pharma Solutions)
- Vitor Sousa (Business Development Manager, Cerbios-Pharma)
- David Cunningham (Director Corporate Development, Goodwin Biotechnology)
- Laurent Ducry (ex-Head of Bioconjugates Commercial Development, Lonza)
- Mark Wright (ex-Site Head, Piramal Pharma Solutions)
- Zhala Tawfiq (Associate Scientist, Ajinomoto Bio-Pharma Services)
- Anonymous (Director, Business Development, Leading CMO)
- Anonymous (Chief Executive Officer, Leading CMO)
The research also includes detailed profiles of key ADC contract manufacturers (listed below); each profile features an overview of the company, its financial information (if available), details on product portfolio, recent developments, and an informed future outlook.
- MabPlex
- AbbVie Contract Manufacturing
- Lonza
- Catalent Pharma Solutions
- Goodwin Biotechnology
- MilliporeSigma
- Piramal Pharma Solutions
- Wuxi Biologics
- Abzena
- CARBOGEN AMCIS
- Cerbios-Pharma
- Formosa Laboratories
- Creative Biolabs
- Novasep
- Sterling Pharma Solutions
To view more details on this report, click on the link
https://www.rootsanalysis.com/reports/view_document/adc-contract-manufacturing-market/218.html
You may also be interested in the following titles:
Decentralized Clinical Trials / Virtual Clinical Trials Market |
About Roots Analysis
Roots Analysis is a global leader in the pharma / biotech market research. Having worked with over 750 clients worldwide, including Fortune 500 companies, start-ups, academia, venture capitalists and strategic investors for more than a decade, we offer a highly analytical / data-driven perspective to a network of over 450,000 senior industry stakeholders looking for credible market insights.
Contact:
Ben Johnson
+1 (415) 800 3415